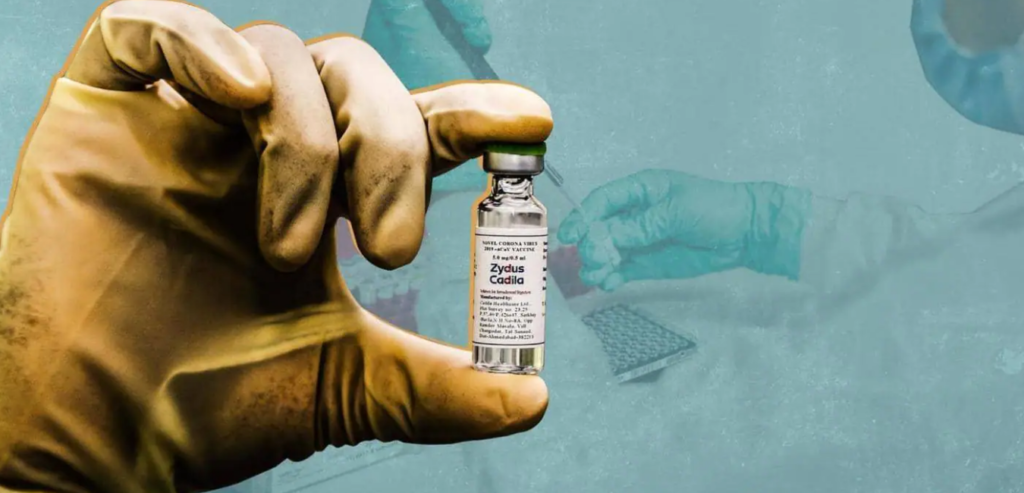
Gujarat-based pharma company Zayedus-Cadila’s three-dose coronavirus vaccine has been approved for emergency use in India on children and adults above the age of 12 years.
This is the first vaccine to be approved for use in India under the age of 18 years.
Built on the Platform DNA Plasmid, it is the world’s first coronavirus vaccine to be approved for use.
This vaccine will be known as Jayakov-D (ZyCoV-D).
Possibly available by October
The vaccine has been developed by the company in association with the Department of Biotechnology, Government of India, and was found to be 66.66 per cent effective in the third phase of trials.
The vaccine has also been approved for use in the age group of 12-18 years, but it is entirely up to the government to decide when it starts vaccination in this age group.
The vaccine is expected to be available for use by October.
These are the qualities of ZyCoV-D
When inside the body, ZyCoV-D will create corona-like spike proteins, which the immune system will identify and start making antibodies. These can be changed according to variants.
Being based on the DNA platform, the vaccine does not require very cold temperatures for storage and costs less.
Further, it does not require highly high biosecurity plant-like Covaccine and other vaccines for its production.
Injecting needles will not be required to get vaccinated
Another important thing about the Zydus Cadila vaccine is that injecting needles will not be required to apply it. It will be an intradermal injection, which is easy to apply.
Sources tell us that intradermal injections are those that are applied to the skin instead of muscles. This also reduces the pain at the time of vaccination.
It is the sixth indigenous vaccine to be approved in India and the second after Covaccine.
3rd dose to be taken on 2nd and 56th day on 28th day
The second dose of the Zydus vaccine will be given on the 28th day of the first dose and the third on the 56th day. The company is also working on a two-dose vaccine. For this, the regulatory body has sought additional data.
Six vaccines approved in India
A total of six corona vaccines including ZyCoV-D have been approved in India, but only three are being used.
Since the inception of the vaccination campaign, Covishield of Serum Institute of India (SII) and Covaccine of Bharat Biotech is being used. The use of Sputnik-V started after getting the green signal in April.
Moderna and Johnson & Johnson vaccines have also been approved, but they have not started to be used.