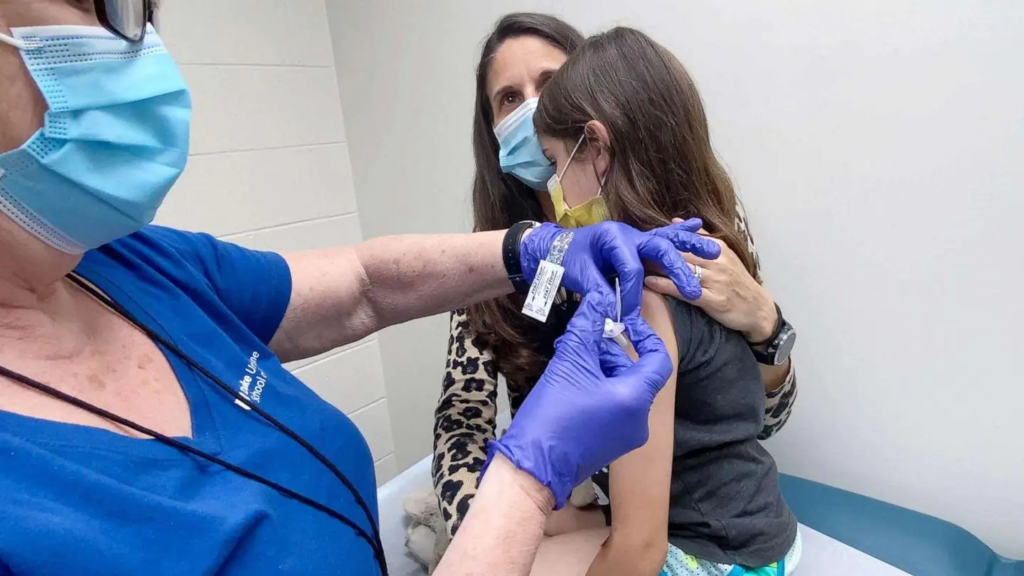
On Tuesday, US pharma company Pfizer said that it would try its coronavirus vaccine on a large group of children under 12.
During the trial, the vaccine will be tested on 4,500 children in 90 places, including the US, Finland, Poland and Spain.
The company hopes that by the end of September, it can get approval for using the Pfizer vaccine on children aged 5-11 years.
Results expected by September
Based on the results of a trial in 144 children in the first phase, Pfizer said it would test the vaccine by giving a dose of 10 micrograms to children aged 5-11 years and three micrograms to children between six months and five years.
A company spokesperson said the trial results involving children aged 5-11 are expected by September. After the results are out, an application will be made for its approval in the same month.
When will the vaccination of children start in India?
Talks are going on between Pfizer and the government to launch the vaccine in India. If Pfizer gets permission to launch here, then the company can launch the vaccine for teenagers. It is believed that from July, Pfizer can start supplying the vaccine in India. On the other hand, vaccine trials on children have begun in the country as well. The results of the test will start coming in between six to nine months.
Why is vaccination of children important?
Vaccination of children is also essential globally, especially in India, because children are feared to be more affected in the third wave of Covid-19. Many experts, including the Scientific Advisor to the Government of India, have said this.
Experts have predicted a third wave after six-seven months, and if the vaccination of children can start in the meantime, then it can be controlled to some extent.